(Cell Death and Cancer Biology Lab)
Research
Research Interest:
Cell death and its regulation, Molecular cancer therapeutics, Anastasis, Drug resistance
Current Research Focus:
Reversal of late-stage apoptosis: Apoptosis, primarily a tumor suppressor process, becomes oncogenic in a context dependent manner. Our lab is working on a novel concept of the reversal of cells from late-stage apoptosis, aka "anastasis". Anastasis plays a contextually specific while complex role in tumorigenesis, disease progression, cancer relapse and metastasis. Through cutting edge technologies and multi-omics approach, we have delineated an extremely significant role of anastasis in oncogenic modification, drug resistance; and the key signaling behind this phenomenon. An in-depth study on its further molecular and physiological characteristics, its communion with cells reversible from other cell death types such as necroptosis, necrosis; and therapeutic drug discovery for targeting these cells form is my short-term career mission.
|
|
Non-canonical apoptosis signaling: Induction of apoptosis is one of the best strategies in cancer treatment. However, aberrant expression of classical apoptotic molecules in cancer cells results in impaired death signal transduction and therefore, leads to failure of therapies. Therefore, deciphering the non-canonical death signaling pathways, for instance bax-bak independent and caspase independent pathways, are indispensable for drug-discovery and better therapeutics. Screening of compounds capable of triggering nonclassical apoptosis signaling or alternative cell death types such as necroptosis, ferroptosis in cancer cells will be prime focus of the lab.
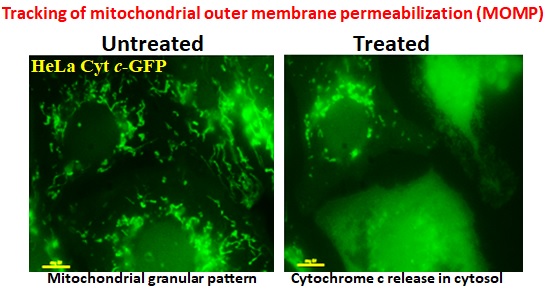 |
Cross-talk of cellular organelles in apoptotic and survival pathways: Mitochondria are the central player in apoptosis process. Cellular stress triggers death signals to promote mitochondrial outer membrane permeabilization (MOMP), a fascinating decision-making event for a cell to die. Interestingly, other organelles such as ER, lysosomes are also emerged as the damage sensors and translating death signals into mitochondria. Therefore, cross-talk between cell organelles is crucial for the modulation of cell death. We are focusing to delineate this important inert-connection between cell organelles in the regulation of cell death. Simultaneously, we are also exploring the emerging role of membrane-less structures, for instance stress-granules, in cell survival and death.